Interactive three-dimensional simulations & visualizations
Visualizing the beauty in physics and mathematics
Project maintained by zhendrikse Hosted on GitHub Pages — Theme by mattgraham
First law of thermodynamics
Theoretical background
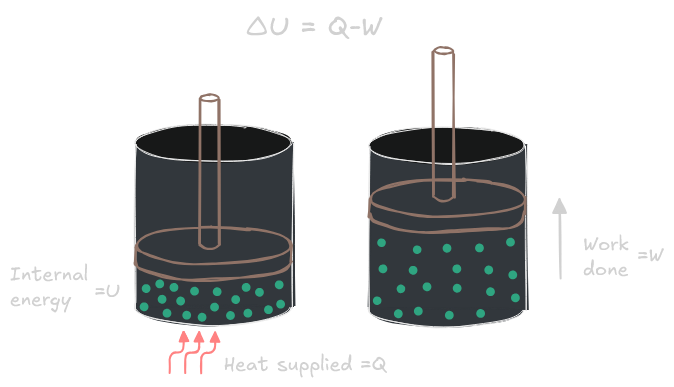
The first law of thermodynamics states that energy cannot be created nor destroyed. It can only be converted from one form into another.
Formulated more rigorously: in a thermodynamic process involving a closed system, the increment in internal energy is equal to the difference between the heat that is either absorbed (or emitted) by the system and the work that is done by it.
Share on: