Interactive three-dimensional simulations & visualizations
Visualizing the beauty in physics and mathematics
Project maintained by zhendrikse Hosted on GitHub Pages — Theme by mattgraham
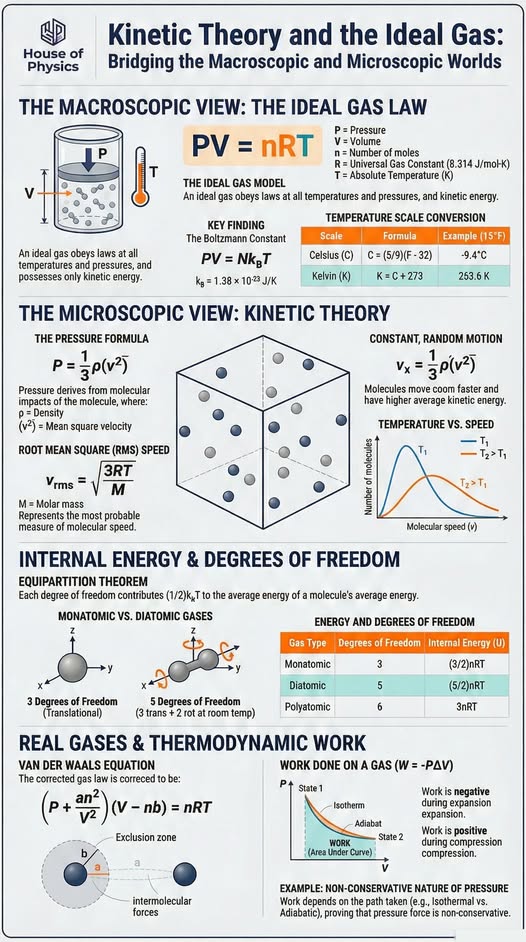
Ideal gas velocity distribution
Theoretical and averaged speed distributions (meters/sec). Initially all atoms have the same speed, but collisions change the speeds of the colliding atoms. One of the atoms is marked and leaves a trail so you can follow its path. The theoretical probability function for the velocities in a tiny volume $dV$ is given by the Maxwell-Boltzmann distribution
$f(\vec{v}) d^3\vec{v} = \bigg(\dfrac{m}{2\pi k_b T} \bigg)^{3/2} \exp \bigg (-\dfrac{mv^2}{2 k_b T} \bigg) d^3\vec{v}$
for a system containing a large number of identical non-interacting, non-relativistic classical particles in thermodynamic equilibrium.
Kinetic theory and the ideal gas
Share on: